Abstract
Introduction Genetic factors may play a role in the development of multiple myeloma (MM), a malignancy of plasma cells. Identification of germline predisposition in MM would have important implications for disease monitoring, familial testing for risk prognostication, and possibly treatment decisions. Here we sought to examine the prevalence of pathogenic germline variants (PGVs) in MM patients using a broad next-generation multi-gene sequencing panel.
Methods We prospectively evaluated patients with MM at various stages of disease (newly diagnosed, relapsed/refractory, etc.) across the Mayo Clinic enterprise (Mayo Clinic Arizona, Mayo Clinic Rochester, Mayo Clinic Florida) between April 1, 2018 and March 31, 2020. Revised international staging system (R-ISS), translocations, and copy number alterations (CNA) were garnered from the electronic health record (EHR). Patients underwent germline testing using an 84-gene next-generation sequencing platform. PGVs were classified as high (relative risk > 4), intermediate (relative risk 2-4), low (relative risk <2) penetrance, or recessive allele. Demographic and clinical characteristics are presented as descriptive statistics. The prevalences of PGVs and variants of unknown significance (VUS) are reported. Categorical variables were compared using the Pearson c2 test. Continuous variables were compared using one-way analysis of variance (ANOVA). P < 0.05 was considered to be statistically significant. All statistical tests were 2-sided.
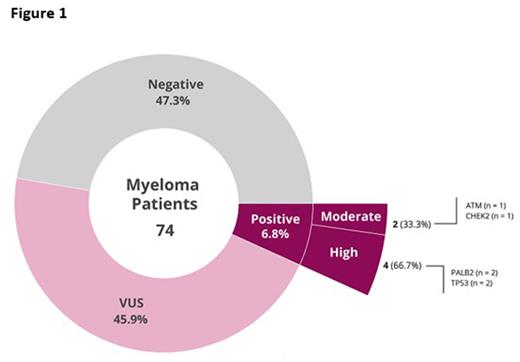
Results A total of 74 patients with MM were evaluated in this cohort. When evaluating the cohort as a whole (regardless of PGV status) the majority of patients had no reported past history of hematologic malignancy (72.7%), but about half of patients (50.7%) had a family history of cancer. Five (6.8%) patients out of the 74 had a PGV, primarily in DNA damage repair genes (Figure 1). Overall, we found a total of 6 PGVs (1 patient with 2 PGVs). Specifically, we found 4 high penetrance mutations, including 2 PALB2 and 2 TP53 mutations, and 2 moderate penetrance mutations, in ATM and CHEK2. We found no significant difference in gender, age, dichotomized race, R-ISS stage, high-risk translocation or CNA, or treatment when comparing patients with PGV, VUS, or negative sequencing results. Median (Q1, Q3) survival differed between groups with 37.4 (34.9,43.7) months in the PGV group, versus 42.3 (30.2, 68.6) months in the VUS group, and 44.4 (31.7, 54.9) months in the groups with negative sequencing results. In this multisite, prospective study of patients with MM at various stages of disease we detected PGVs at a prevalence of 6.8%. The majority of germline variants we identified were in DNA damage repair genes.
Conclusion Here, we show that multigene panel testing can effectively detect PGVs in a substantial group of patients with MM that can have implications for future cancer risk screening both for the patient and their family members. A weakness of our study is our relatively small cohort size. Although we did not find an association between family history of hematologic malignancy, translocation, CNA or differences in survival, this is likely due in part to the size of the cohort. When evaluating median follow-up we saw shorter survival times in the PGV group. Larger prospective studies should be conducted on patients with MM at different stages of disease to assess the prevalence of germline alterations and association with disease characteristics, prognosis and implications for future cancer screening.
Disclosures
Esplin:Invitae: Current Employment, Current holder of stock options in a privately-held company; Taproot Health: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees. Nussbaum:Invitae: Current Employment, Current holder of stock options in a privately-held company; Genome Medical: Consultancy, Current holder of stock options in a privately-held company; Maze Therapeutics: Consultancy, Current holder of stock options in a privately-held company; Pfizer: Consultancy. Stewart:GlaxoSmithKline: Consultancy; Sanofi: Consultancy; Tempus Health: Consultancy, Other: Stock ownership (not including stocks owned in a managed portfolio; Janssen: Consultancy; Amgen: Consultancy. Samadder:Jansen Research: Consultancy; Cancer Prevention Pharma: Consultancy; Recursion Pharmaceutical: Consultancy. Bergsagel:Oncopeptides: Consultancy; Janssen: Consultancy; GSK: Consultancy; Novartis: Consultancy; Pfizer: Consultancy. Fonseca:Juno: Consultancy; Karyopharm: Consultancy; Bayer: Consultancy; Sanofi: Consultancy; Regeneron: Consultancy; Pharmacyclics: Consultancy; Pfizer: Consultancy; OncoTracker: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Consultancy; Novartis: Consultancy; Merck: Consultancy; Kite: Consultancy; Takeda: Consultancy; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; H3 Therapeutics: Consultancy; GSK: Consultancy; BMS/Celgene: Consultancy; Amgen: Consultancy; AbbVie: Consultancy; Caris Life Sciences: Membership on an entity's Board of Directors or advisory committees; OncoMyx: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal